Transcranial magnetic stimulation to treat 101 patients with depression and comorbid personality disorders in a real-world naturalistic clinical setting: feasibility, tolerability and effectiveness
Highlights
Abstract
Introduction
Many patients with depression fail to achieve adequate response or remission with standard treatments, prompting an urgent need to identify factors influencing treatment outcomes. Among these, comorbid personality disorders (PDs) have been identified as a significant contributor to poorer treatment responses.
Methods and materials
In this open-label, naturalistic study, we evaluated the feasibility, tolerability, and effectiveness of intermittent theta burst stimulation (iTBS) in a sample of 101 patients with depression and comorbid PD. Eighty-six patients received a standard iTBS protocol (one session per day over several weeks), while fifteen opted for an accelerated protocol involving two to four sessions per day. Symptom severity was assessed using the Beck Depression Inventory (BDI) and the Symptom Checklist-90-R (SCL-90-R), administered at baseline, weekly during treatment, and at four follow-up points (1, 2, 3, and 4 months post-treatment).
Results and conclusion
Both treatment protocols led to significant symptom reduction, which was sustained over the follow-up period. Remission rates were 87 % in the accelerated group and 63 % in the standard protocol group. iTBS was well tolerated, with no serious adverse events reported. Notably, patients who were not taking antidepressant medication and those who were younger showed better treatment responses. iTBS appears to be a feasible, safe, and effective treatment option for depressed patients with comorbid PD. The findings suggest the benefits of iTBS treatment and support further exploration of patient characteristics that may predict response to rTMS.
1. Introduction
2. Method
2.1 Procedure
Our sample consisted exclusively of outpatients who had received an MDD diagnosis with comorbid PD. All patients provided informed consent at the Medical Psychotherapeutic Center (ΙΨΚ) in Thessaloniki, Greece before undergoing treatment. The treatment sessions were conducted at ΙΨΚ between 2018 and 2023. Considering that this was an open-label naturalistic study in a clinical population sample, we were unable to control for variables such as age, sex, medication use and psychotherapy before treatment, and therefore included those as potential covariates in our statistical analyses.
2.2 Participants
2.2.1 Eligibility criteria
2.2.2 Sample demographics
| Characteristic | Standard protocol (N = 86) | Accelerated protocol (N = 15) | All patient sample (N = 101) |
|---|---|---|---|
| Age (M/SD) | 42.98 (14.88) | 43.06 (15.10) | 43.00 (14.84) |
| Sex (males/females) | 45/41 | 11/4 | 56/45 |
| Baseline BDI score (M/SD) | 28.23 (10.02) | 23.60 (8.45) | 27.54 (9.90) |
| No. of sessions (M/SD) | 25.23 (5.91) | 24.13 (5.46) | 25.06 (5.83) |
| Mean TMS dose (120%) (M/SD) | 44.14 (7.88) | 44.46 (8.79) | 44.18 (7.98) |
| Antidepressant medication type |
No. of patients taking medication in the standard iTBS protocol | No. of patients taking medication in the accelerated iTBS protocol | Total No. of patients |
|---|---|---|---|
| SSRI or SNRI | 26 | 5 | 31 |
| SSRI or SNRI augmented by other class (e.g., antipsychotics, mood stabilisers) | 22 | 3 | 25 |
| Other (e.g., antipsychotics, mood stabilisers) | 10 | 0 | 10 |
| Subtotal | 58 | 8 | 66 |
| None | 28 | 7 | 35 |
| TOTAL | 86 | 15 | 101 |
Table 2 Classes of medication prescribed for depression for the patient sample that comprised the standard and accelerated protocols, and the overall patient sample.
| Personality Disorder | All patients | No. of patients with PD type for the standard iTBS protocol | No. of patients with PD type for the accelerated iTBS protocol |
|---|---|---|---|
| Paranoid | 0 | 0 | 0 |
| Schizoid | 1 | 0 | 0 |
| Schizotypal | 1 | 1 | 0 |
| Antisocial | 0 | 0 | 0 |
| Borderline | 3 | 2 | 1 |
| Histrionic | 10 | 8 | 2 |
| Narcissistic | 2 | 2 | 0 |
| Avoidant | 3 | 3 | 0 |
| Dependent | 1 | 1 | 0 |
| Obsessive-Compulsive | 1 | 2 | 0 |
| Mixed | 79 | 67 | 12 |
table {
width: 100%;
border-collapse: collapse;
}
th, td {
padding: 8px 12px;
text-align: left;
border: 1px solid #ddd;
}
th {
background-color: #f4f4f4;
}
/* Ensuring the table stays responsive */
@media (max-width: 768px) {
.table-wrap {
-webkit-overflow-scrolling: touch;
overflow-x: scroll;
}
table {
min-width: 600px; /* You can adjust the min-width based on your content */
}
}
Table 3
Distribution of personality disorders for all patients and by protocol type.
| iTBS Protocol | Response | Remission |
|---|---|---|
| Accelerated (N = 15) Males (N = 11) Females (N = 4) | 80% 73% 100% | 87% 82% 100% |
| Standard (N = 86) Males (N = 45) Females (N = 41) | 57% 64% 49% | 63% 66% 55% |
| All patients (N = 101)* | 64% | 66% |
| PD cluster | ||
| Cluster A (N = 42) | 61% | 64% |
| Cluster B (N = 67) | 60% | 63% |
| Cluster C (N = 77) | 58% | 62% |
table {
width: 100%;
border-collapse: collapse;
}
th, td {
padding: 8px 12px;
text-align: left;
border: 1px solid #ddd;
}
th {
background-color: #f4f4f4;
}
/* Ensuring the table stays responsive */
@media (max-width: 768px) {
.table-wrap {
-webkit-overflow-scrolling: touch;
overflow-x: scroll;
}
table {
min-width: 600px; /* You can adjust the min-width based on your content */
}
}
Table 4
Response and remission rates based on BDI scores at end of treatment, by iTBS protocol, sex, and PD cluster.
Note:
* Includes all patients treated with iTBS, regardless of protocol type (standard or accelerated).
2.3 Materials
2.3.1 TMS
2.3.2 iTBS protocol
Each intermittent theta-burst stimulation (iTBS) session consisted of 600 pulses, delivered in bursts of three pulses at 50 Hz, repeated at a frequency of 5 Hz. These bursts were administered in 2-second trains, followed by 8-second inter-train intervals, for a total of 20 trains per session. This protocol is consistent with standard clinical iTBS procedures. All patients (N = 101) were offered a choice between a standard (non-accelerated) and an accelerated iTBS protocol, depending on personal preference and logistical considerations such as work obligations or travel distance. The majority of patients (86 out of 101) chose the standard protocol, receiving one session per weekday over a period of 4–6 weeks, resulting in a total of 20–30 sessions. Fifteen patients opted for the accelerated protocol. Among these, 11 received two sessions per day with a 50-minute break in between, over a period of 2–3 weeks, resulting in a total of 20–30 sessions. Four patients, who were living abroad, completed four sessions per day with 50-minute breaks between each, over a period of one week to 2 weeks resulting in a total of 20–40 sessions.
2.3.3 Beck depression inventory
2.3.4 Symptom checklist 90-R
2.3.5 International personality disorder examination (IPDE)
2.4 Statistical analysis
A linear mixed model (LMM) analysis was used to assess iTBS treatment effects on depression levels over time, measured by BDI scores and the depression subscale of the SCL-90-R. Time (pre-treatment vs. post-treatment) was a within-subject factor, while Protocol Type (accelerated vs. standard) was a between-subject factor. Covariates included: sex, age, total number of classes of medications, number of benzodiazepines, adjunct psychotherapy, TMS dosage (i.e., stimulation intensity), and number of sessions. As our data lacked independency, in view of our repeated measures design, and due to the fact that the two protocol groups were unbalanced, we chose LMM as the preferred analysis method. LMMs can model unequal sample sizes and are also appropriate for continuous outcomes, while they allow for random effects, accounting for unexplained variance within our patient sample. Data was normally distributed as skewness and kurtosis for both BDI (skewness =.59; kurtosis = −.29). and SCL-90-R (SCL-90-R skewness =.52; kurtosis =.15) were within normal ranges. Q-Q plots indicated that both assumptions of normality and linearity were met for BDI and SCL-90-R. PD effects were analyzed through four models examining PD clusters and individual PD diagnoses. A backwards selection process simplified the initial comprehensive model, retaining relevant fixed factors; PD clusters (centered); and interaction terms involving Time, Protocol, and PDs. Individual PD models included centered PD diagnoses and interaction terms up to four-way interactions, with BPD and AVDPD prioritized due to prevalence. Antisocial PD was excluded due to insufficient observations. Post-hoc Bonferroni-corrected pairwise comparisons identified time points of significant changes between protocols (p
3. Results
3.1 Safety and tolerability
3.2 Practical feasibility
3.3 Response and remission rates
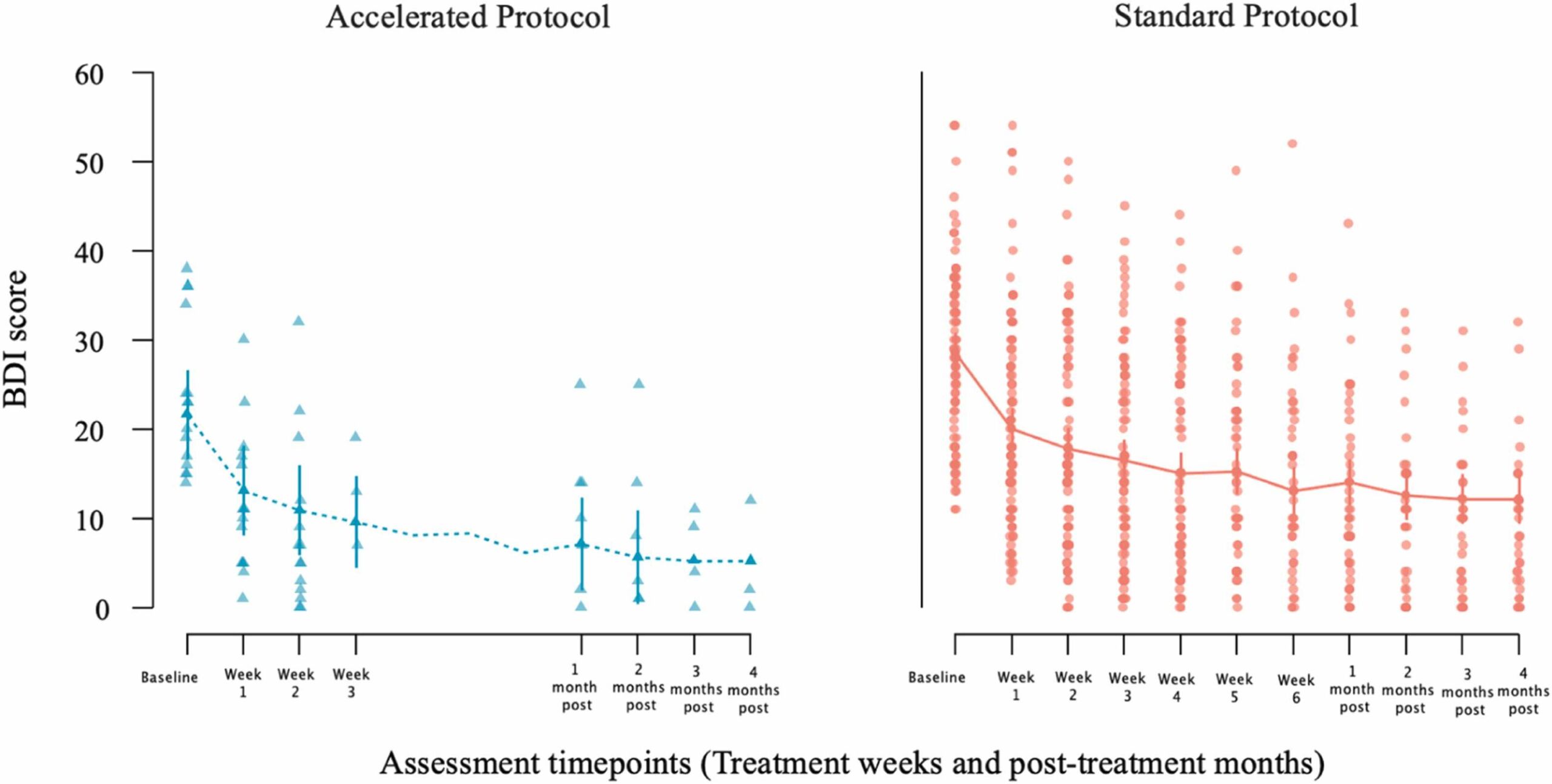
Fig. 1 Change in patients’ BDI scores from baseline to end of treatment [week 3 for the accelerated group (left panel); week 6 for the standard group (right panel)] and from baseline to post-treatment follow-up month (first through fourth post months) assessments after the end of each patient treatment. Dots represent individual patient scores; lines indicate group means with standard error bars.
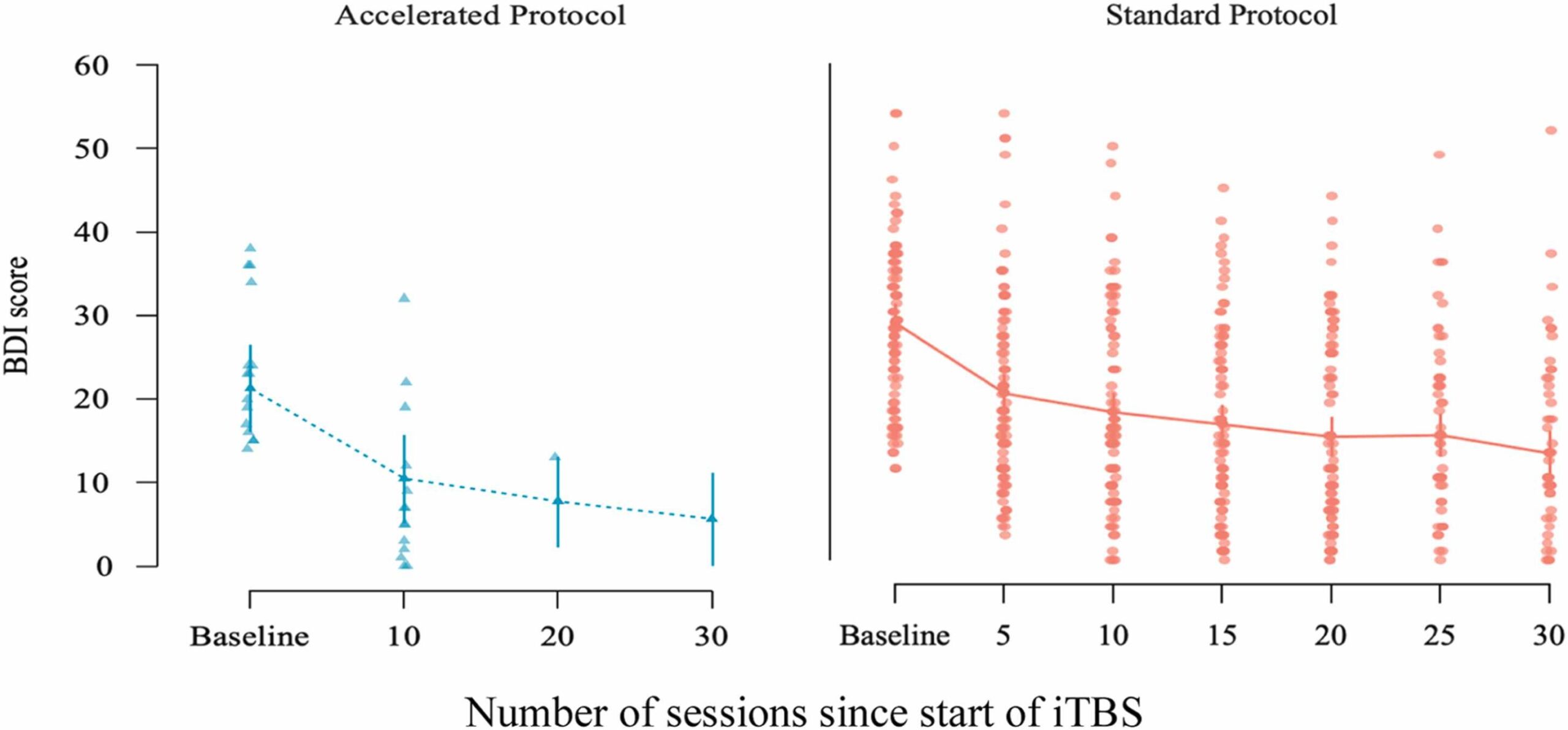
Fig. 2 Change in BDI scores across treatment sessions for the accelerated and standard iTBS protocols, shown separately. The x-axis reflects the number of sessions since start of treatment. The accelerated protocol group (left panel) was assessed at baseline and after every 10 sessions (sessions 10, 20, 30). The standard protocol group (right panel) was assessed at baseline and every 5 sessions. Dots represent individual patient scores; lines indicate group means with standard error bars.
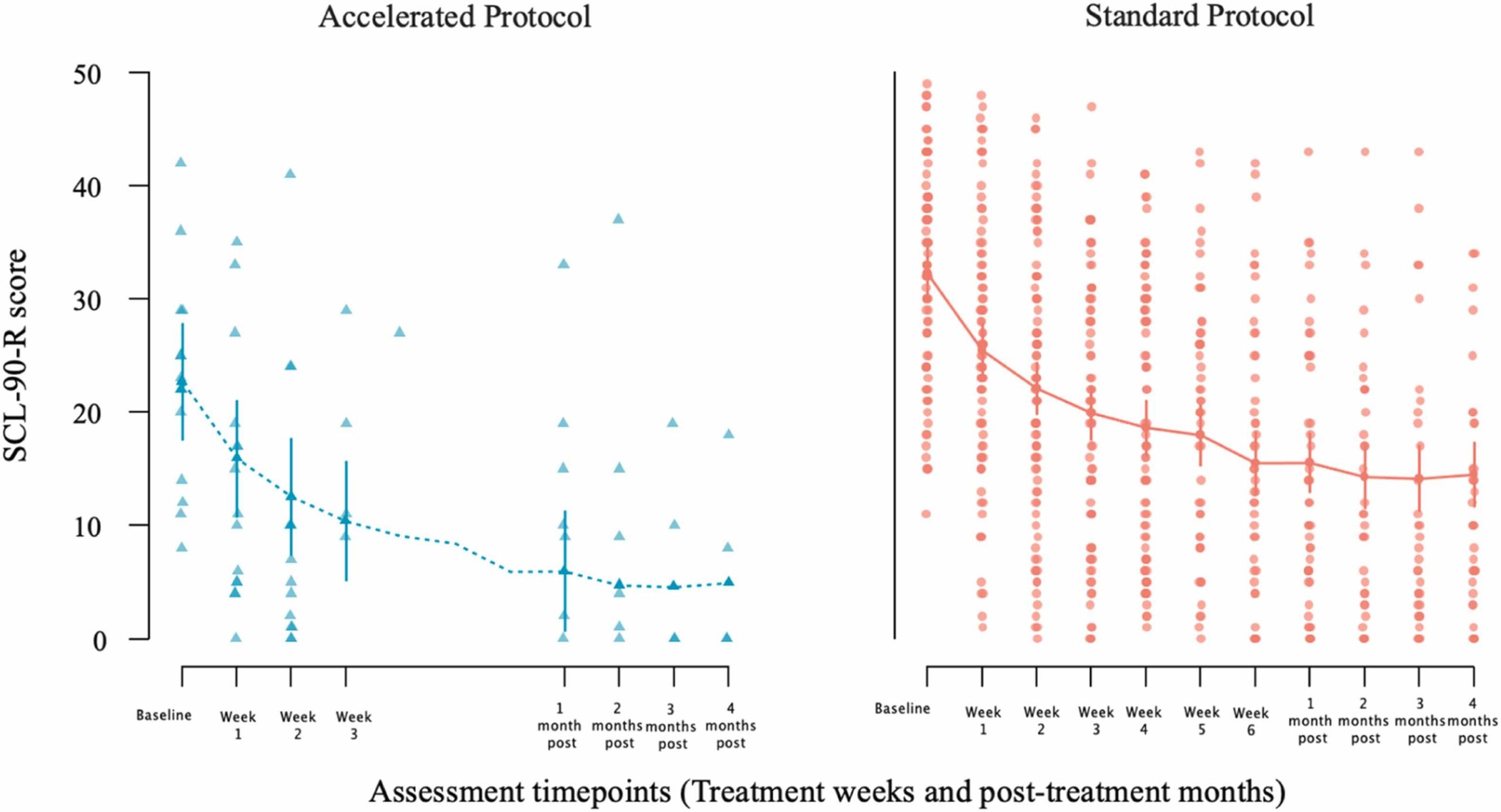
Fig. 3 Change in patients’ SCL-90-R scores from baseline to end of treatment [week 3 for the accelerated group (left panel); week 6 for the standard group (right panel)] and from baseline to post-treatment follow-up month (first through fourth post months) assessments after the end of each patient treatment. Dots represent individual patient scores; lines indicate group means with standard error bars.
3.4 BDI outcomes: effects of iTBS treatment
3.4.1 Model 1: PD clusters
| Fixed Effect | df | X2 | p |
|---|---|---|---|
| Time | 10 | 369.275 | < .001** |
| Protocol type | 1 | 4.833 | .028* |
| Medications | 6 | 15.596 | .016* |
| Cluster A | 1 | 0.077 | .781 |
| Cluster B | 1 | 0.079 | .779 |
| Cluster C | 1 | 0.799 | .372 |
table {
width: 100%;
border-collapse: collapse;
}
th, td {
padding: 8px 12px;
text-align: left;
border: 1px solid #ddd;
}
th {
background-color: #f4f4f4;
}
/* Ensuring the table stays responsive */
@media (max-width: 768px) {
.table-wrap {
-webkit-overflow-scrolling: touch;
overflow-x: scroll;
}
table {
min-width: 600px; /* You can adjust the min-width based on your content */
}
}
3.4.2 Model 2: specific PD diagnoses
| Fixed Effect | df | X2 | p |
|---|---|---|---|
| Time | 7 | 195.053 | < .001** |
| Medications | 6 | 14.488 | .025* |
| Time*BPD | 7 | 9.072 | .248 |
| Protocol*Time | 7 | 9.736 | .204 |
| Protocol*Time*BPD | 7 | 20.402 | .005* |
table {
width: 100%;
border-collapse: collapse;
}
th, td {
padding: 8px 12px;
text-align: left;
border: 1px solid #ddd;
}
th {
background-color: #f4f4f4;
}
/* Ensuring the table stays responsive */
@media (max-width: 768px) {
.table-wrap {
-webkit-overflow-scrolling: touch;
overflow-x: scroll;
}
table {
min-width: 600px; /* You can adjust the min-width based on your content */
}
}
3.5 SCL-90-R outcomes: effects of iTBS treatment
3.5.1 Model 1: PD clusters
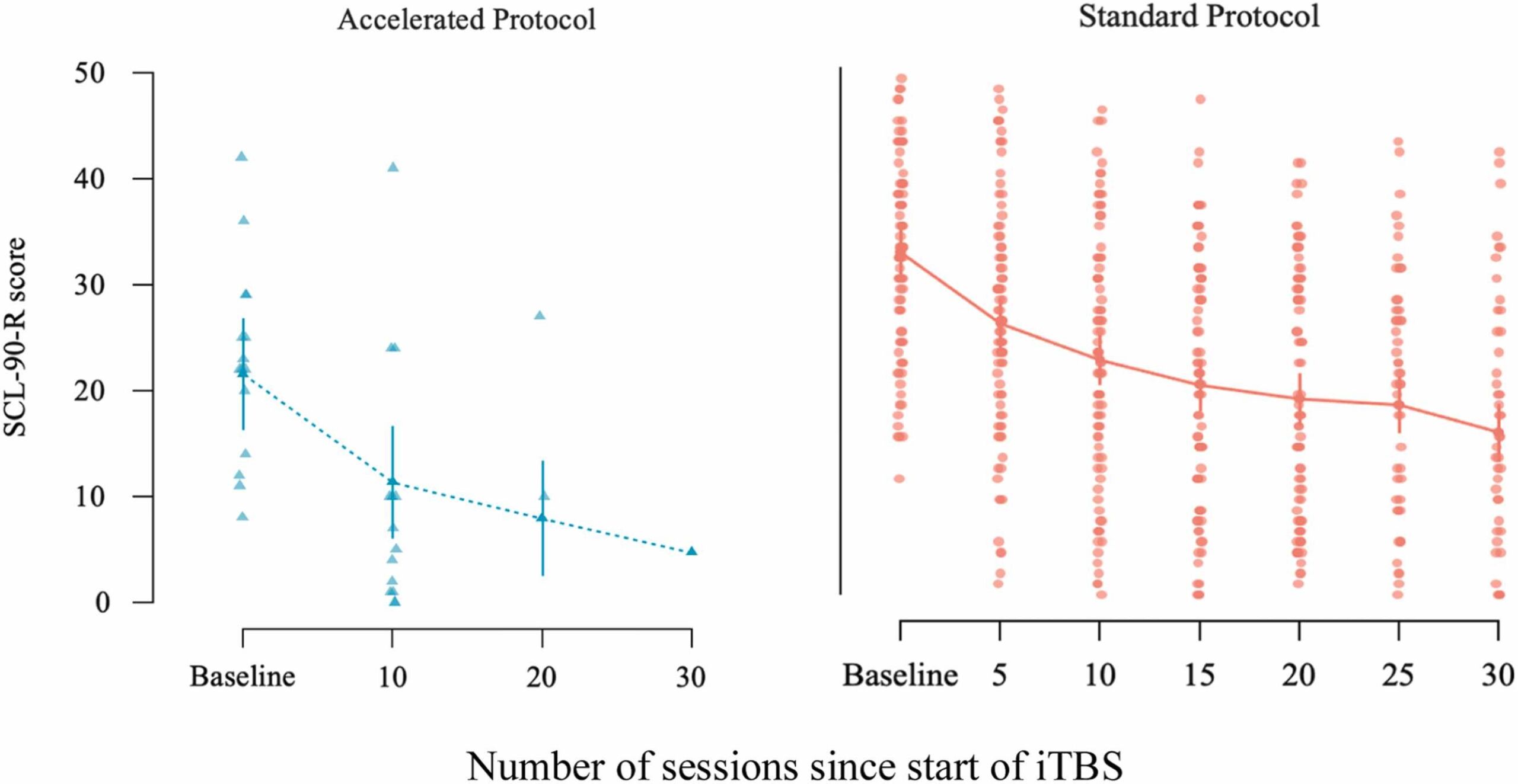
Fig. 4 Change in SCL-90-R scores across treatment sessions for the accelerated and standard iTBS protocols, shown separately. The x-axis reflects the number of sessions since start of treatment. The accelerated protocol group (left panel) was assessed at baseline and after every 10 sessions (sessions 10, 20, 30). The standard protocol group (right panel) was assessed at baseline and every 5 sessions. Dots represent individual patient scores; lines indicate group means with standard error bars.
| Fixed Effect | df | X2 | p |
|---|---|---|---|
| Time | 10 | 391.093 | < .001** |
| Protocol type | 1 | 9.849 | .002* |
| Medications | 6 | 16.340 | .012* |
| Age | 1 | 7.668 | .006* |
| Cluster C | 1 | 5.345 | .021* |
table {
width: 100%;
border-collapse: collapse;
}
th, td {
padding: 8px 12px;
text-align: left;
border: 1px solid #ddd;
}
th {
background-color: #f4f4f4;
}
/* Ensuring the table stays responsive */
@media (max-width: 768px) {
.table-wrap {
-webkit-overflow-scrolling: touch;
overflow-x: scroll;
}
table {
min-width: 600px; /* You can adjust the min-width based on your content */
}
}
3.5.2 Model 2: specific PD diagnoses
| Fixed Effect | df | X2 | p |
|---|---|---|---|
| Time | 8 | 166.036 | < .001** |
| Medications | 6 | 15.351 | .018* |
| Age | 1 | 6.641 | .010* |
| AvD PD | 1 | 3.833 | .050 |
| Time*AvD | 10 | 20.135 | .028* |
| Time*BPD*Protocol | 8 | 17.082 | .029* |
table {
width: 100%;
border-collapse: collapse;
}
th, td {
padding: 8px 12px;
text-align: left;
border: 1px solid #ddd;
}
th {
background-color: #f4f4f4;
}
/* Ensuring the table stays responsive */
@media (max-width: 768px) {
.table-wrap {
-webkit-overflow-scrolling: touch;
overflow-x: scroll;
}
table {
min-width: 600px; /* You can adjust the min-width based on your content */
}
}
Post-hoc comparisons revealed a reduction of 15.89 points (66.3 %) in SCL-90-R scores at Time 8 compared to baseline (z = 10.016, p < .001), and a 15.88-point reduction (46.3 %) at the two-month follow-up (z = 9.365, p < .001; Figs. 4 and 5). Patients taking four types of medication scored 8.57 points (92.2 %) higher than those not on any medication (z = 2.937, p = .017). Younger patients reported significantly higher SCL-90-R scores than older individuals, with the youngest group scoring 2.19 points more than the middle-aged group (z = 2.618, p = .027) and 4.39 points more than the oldest group.
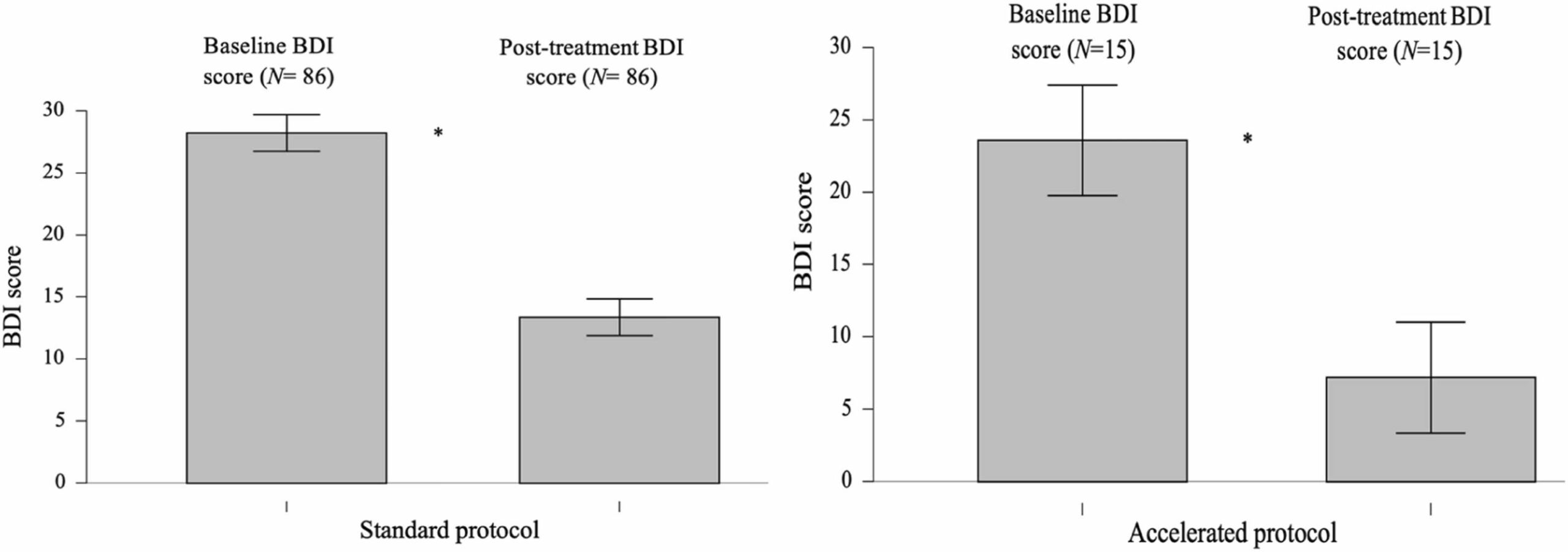
Fig. 5 Bar plots of BDI scores at pre-treatment and post-treatment for the standard (left) and accelerated protocols (right). Whiskers indicate the full data range. Asterisks denote statistical significance.
3.6 Impact of personality disorder traits on treatment efficacy
3.7 Protocol group differences in pre- and post-treatment BDI scores
| PD Clusters/PDs | Pre-treatment (M, SD) | Post-treatment (M, SD) | % Mean Difference | df | t | p |
|---|---|---|---|---|---|---|
| Cluster A | 30.71 (10.05) | 15.71 (12.50) | 48.84 | 23 | 6.808 | < .004 |
| Cluster B | 29.71 (10.10) | 14.86 (10.86) | 49.98 | 27 | 8.178 | < .001 |
| Cluster C | 30.49 (9.16) | 15.54 (11.59) | 49.03 | 36 | 8.532 | < .001 |
| Paranoid | 30.31 (10.12) | 18.13 (13.99) | 40.18 | 15 | 4.442 | < .001 |
| Schizoid | 31.18 (8.14) | 15.82 (9.85) | 49.26 | 16 | 5.671 | < .001 |
| Schizotypal | 29.78 (7.59) | 14.56 (8.35) | 51.10 | 8 | 4.069 | .004 |
| Histrionic | 27.42 (10.09) | 9.75 (8.23) | 64.44 | 11 | 6.522 | < .001 |
| Narcissistic | 30.67 (14.67) | 14.67 (15.70) | 52.16 | 8 | 3.913 | .004 |
| Borderline | 31.27 (10.36) | 16.14 (10.93) | 48.38 | 21 | 6.742 | < .001 |
| Antisocial | 23.33 (1.52) | 11.33 (10.06) | 51.43 | 2 | 2.110 | .169 |
| OCPD* | 28.79 (8.66) | 10.54 (7.49) | 63.39 | 23 | 8.955 | < .001 |
| Dependent | 30.42 (11.61) | 15.47 (11.87) | 49.14 | 18 | 5.969 | < .001 |
| Avoidant | 30.41 (8.80) | 15.75 (11.61) | 48.20 | 31 | 7.629 | < .001 |
table {
width: 100%;
border-collapse: collapse;
}
th, td {
padding: 8px 12px;
text-align: left;
border: 1px solid #ddd;
}
th {
background-color: #f4f4f4;
}
/* Ensuring the table stays responsive */
@media (max-width: 768px) {
.table-wrap {
-webkit-overflow-scrolling: touch;
overflow-x: scroll;
}
table {
min-width: 700px; /* Adjusted for better display on smaller screens */
}
}
4. Discussion
4.1 Limitations and future research
5. Conclusions
Our open-label naturalistic study assessed the feasibility, tolerability, and effectiveness of a repetitive transcranial magnetic stimulation in 101 patients with depression and comorbid PD. The findings indicate that both the standard and accelerated protocols are feasible, well-tolerated and clinically effective in this population. Larger studies are needed to confirm the effectiveness of the accelerated protocol. Given that iTBS requires less time than conventional rTMS protocols, it offers greater flexibility and reduced time burden for both patients and clinicians. These promising results underscore the importance of follow-up research using randomized controlled trial designs.
CRediT authorship contribution statement
Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Panagiota Koutsimani: Writing – review & editing, Writing – original draft, Visualization, Formal analysis. Teresa Schuhmann: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Project administration, Methodology, Investigation, Formal analysis, Data curation. Alexander T. Sack: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Formal analysis, Conceptualization.
Ethics statement
Funding
This research did not receive external funding.
Declaration of Competing Interest
Acknowledgements
We would like to thank all the psychologists and psychiatrists at the Medical Psychotherapeutic Centre in Thessaloniki, Greece, who referred patients for this study.
Appendix A Supplementary material (1)
Data Availability
References




